|
|
"HEB" Hemispherx BioPharma.... 第二帖
[复制链接]
|
|
|
 发表于 9-10-2009 07:43 AM
|
显示全部楼层
发表于 9-10-2009 07:43 AM
|
显示全部楼层
Study Isolates Virus in Chronic Fatigue Sufferers
Thursday, 08 October 2009 11:38
ABC News is reporting that a virus linked to prostate cancer also appears to play a role in chronic fatigue syndrome (CFS), this according to research that could lead to drug treatments for a mysterious disorder that affects 17 million people worldwide. Prior this news, BioMedReports had scheduled an interview with Dr. William A. Carter, CEO of Hemispherix Biopharma (AMEX:HEB), whose company is still waiting for approval for it's CFS treatment. That report/interview will appear on BioMedReports during market hours on Friday.
According to the breaking news from Reuters, researchers found the virus, known as XMRV, in the blood of 68 out of 101 chronic fatigue syndrome patients. The same virus showed up in only 8 of 218 healthy people, they reported on Thursday in the journal Science.
Dr. William A. Carter, CEO of Hemispherx BioPharma, Inc. (AMEX:HEB) is expected to share some important news about the company's pending FDA decision for Ampligen as a first line treatment for Chronic Fatigue Syndrome (CFS) and some new information about the company's Alferon LDO (Low Dose Oral) application.
The Company’s products include Ampligen and Alferon N and Injection. Ampligen includes application as a treatment for Chronic Fatigue Syndrome (CFS) and as a vaccine enhancer(adjuvant) for both therapeutic and preventative vaccine development. Alferon N is an indicator for refractory or recurring genital warts. Alferon LDO (Low Dose Oral) is an application under early stage development targeting influenza and viral diseases. Ampligen is an experimental drug undergoing clinical development for the treatment of CFS.
CFS impairs the immune system and causes incapacitating fatigue, according to the U.S. Centers for Disease Control and Prevention. Sufferers can also experience memory loss, problems with concentration, joint and muscle pain, headaches, tender lymph nodes and sore throats.
Symptoms last at least six months and can be as disabling as multiple sclerosis or rheumatoid arthritis, the CDC said. |
|
|
|
|
|
|
|
|
|
|
|
 发表于 15-10-2009 08:57 PM
|
显示全部楼层
发表于 15-10-2009 08:57 PM
|
显示全部楼层
Japanese NIH Research Reaffirms and Expands Pandemic Flu Protection by Ampligen(R)
October 15, 2009 8:15 AM EDT
PHILADELPHIA, Oct. 15, 2009 (GLOBE NEWSWIRE) -- Hemispherx Biopharma, Inc. (NYSE Amex: HEB) (the "Company"), announced that Dr. H. Hasegawa, Chief, Laboratory of Mucosal Vaccine Development Virus Research Center, Japanese National Institute of Infectious Diseases (JIID), expanded the data on Ampligen(R), an experimental immunotherapeutic, at the "Mucosal Immunity" session of the Japan-France Vaccine and Infectious Diseases Workshop in Osaka, October 10, 2009. (Please see http://www.osaka-u.ac.jp/en/seminar/info/2009/10/594). Dr. Hasegawa is the Principal Investigator on the Ampligen(R)/ Influenza vaccine program under the joint auspices of JIID/ Ministry of Health, Biken Corporation (Osaka) and Hemispherx Biopharma. Dr. Hasegawa provided an overview of intranasal pandemic flu vaccine (H5N1) and nasal immunity mechanisms.
The new data consist of both expanded clinical and anatomical findings in macaque monkeys exposed to the most virulent forms of pandemic influenza (H5N1). Standard human seasonal influenza vaccines -- given alone and having no benefit on H5N1 influenza virus pathology and clinical status -- were nonetheless highly effective against pandemic virus when combined with Ampligen(R) (Poly I: Poly C12U), an experimental therapeutic, which was applied intranasally in very small doses, in a prophylactic treatment setting.
According to various sources, pandemic influenza vaccines will be in very limited supply on a global basis. Thus, many countries are not expected to be able to access significant pandemic vaccines stock piles. In contrast, the sources of seasonal human influenza vaccines are much more abundant and readily accessible. Hemispherx's goal is to expand the observations of JIID into other countries including the South American continent where relevant clinical vaccination protocols are being actively developed for the current pandemic H1N1 influenza virus. According to WHO's (World Health Organization) Initiative for Vaccine Research, in some countries only 10% of the population will have access to pandemic vaccine.
According to Dr. Hasegawa's research including that recently published at the Sapporo, Japan, Vaccinology Conference, Ampligen(R) (Poly I : Poly C12U), an experimental immunotherapeutic and centerpiece of a mucosal immunity program, may convey two additional biological properties (in addition to the above referenced cross-protection) when co-administered intranasally with pandemic flu vaccines: 1) the enhancement of immunity with higher IgA and IgG levels which may convey a survival/therapeutic advantage in animal model systems, and 2) the potential to widen the therapeutic (preventative) profile by protecting against a phenomenon known as "antigenic drift" in which the pandemic virus may escape the preventative effect of the vaccine; this phenomenon is well-established with avian H5N1 virus and mitigated the potential effectiveness of various influenza vaccines manufactured several years ago in the U.S.A.
A description of the vaccine manufacturing technology can be accessed on the Hemispherx web site, announcement of October 8, 2009.
Avian influenza (H5N1), endemic in the Pacific Rim countries, has a greater than 60% death rate in human transmissions to date while the current swine flu (H1N1) death rate is much lower (please see www.flucount.org: swine flu count- world wide statistics of the H1N1 Influenza Pandemic). Moreover, H5N1 requires only a mutation to allow primary recognition of human cell receptors for easy transmission. The research in the monkey model suggests that even when the lethality genes are concentrated in the influenza virus (e.g., avian H5N1), Ampligen(R) (Poly I: Poly C12U), an experimental therapeutic, continues to conveys an effective preventative regimen.
Animal model experiments do not necessarily predict biological behavior in man. Regulatory agencies are the only governmental entities vested with the authority to determine whether biological products and experimental therapeutics may be deemed safe and effective for use in a human population.
http://www.streetinsider.com/Press+Releases/Japanese+NIH+Research+Reaffirms+and+Expands+Pandemic+Flu+Protection+by+Ampligen(R)/5018790.html |
|
|
|
|
|
|
|
|
|
|
|

楼主 |
发表于 18-10-2009 07:47 PM
|
显示全部楼层
Hemispherx Biopharma, Inc. (HEB) Support & Resistance Levels - 10/19/09
Resistance Levels: $1.90, $2.16
Support Levels: $1.75, $1.64
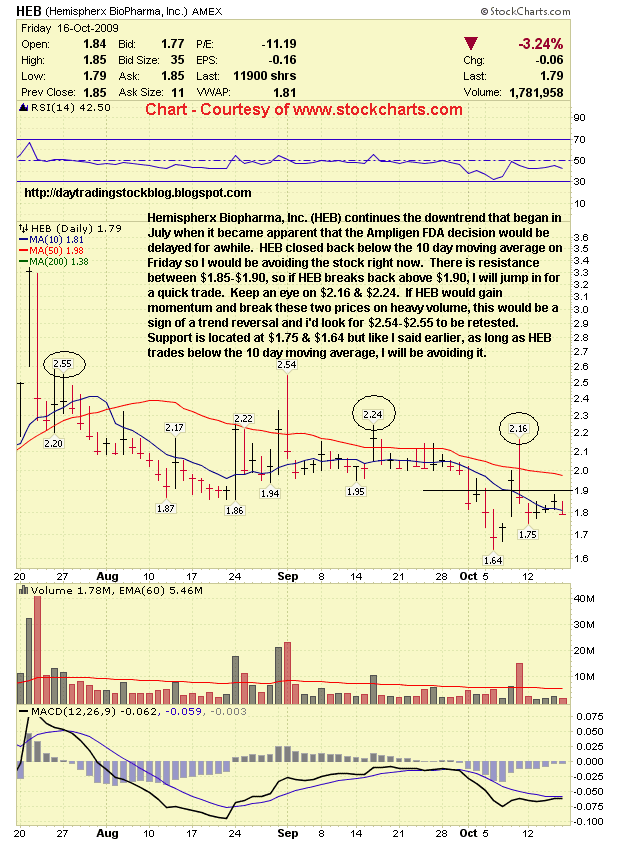
Hemispherx Biopharma, Inc. (HEB) continues the downtrend that began in July when it became apparent that the Ampligen FDA decision would be delayed for awhile. HEB closed back below the 10 day moving average on Friday so I would be avoiding the stock right now. There is resistance between $1.85-$1.90, so if HEB breaks back above $1.90, I will jump in for a quick trade. Keep an eye on $2.16 & $2.24. If HEB would gain momentum and break these two prices on heavy volume, this would be a sign of a trend reversal and I'd look for $2.54-$2.55 to be retested. Support is located at $1.75 & $1.64 but like I said earlier, as long as HEB trades below the 10 day moving average, I will be avoiding it. |
|
|
|
|
|
|
|
|
|
|
|

楼主 |
发表于 22-10-2009 06:13 PM
|
显示全部楼层
CTIC 卡帖卡的太厲害了。。。 借用HEB帖。。。
Cell Therapeutics Says Pixantrone Studies Show High Rates Of Complete Remission In Relapsed/refractory Aggressive, Indo
Cell Therapeutics Says Pixantrone Studies Show High Rates Of Complete Remission In Relapsed/refractory
Aggressive, Indolent NHL Patients - Quick Facts
Thu Oct 22 02:07:00 2009
EDT
(RTTNews) - Cell Therapeutics, Inc. (CTIC) announced that Richard Van der Jagt of the Ottawa General
Hospital will present at the Lymphoma and Myeloma 2009 Conference in New York an overview of the company's
pixantrone phase II and phase III clinical studies that demonstrated high rates of complete remission
in relapsed/refractory aggressive and indolent non-Hodgkin's lymphoma, or NHL, patients.
The company revealed 70% CR/CRu rate in a phase II trial in patients treated with pixantrone plus
FPD-R regimen in relapsed/refractory indolent NHL. The company also announced 47% CR/CRu rate in a
phase II trial in patients treated with CPOP regimen for patients that failed CHOP regimen with relapsed/refractory
aggressive NHL. Cell Therapeutics further disclosed 35% CR rate in a phase III trial in patients treated
with pixantrone plus rituximab in relapsed/refractory indolent NHL.
For comments and feedback: contact editorial@rttnews.com
Copyright(c) 2009 RTTNews.com, Inc. All Rights Reserved
CTIC,
BREAKING_NEWS, US, CORPORATE_NEWS, |
|
|
|
|
|
|
|
|
|
|
|

楼主 |
发表于 23-10-2009 03:47 PM
|
显示全部楼层
23/10/09
Hemispherx Biopharma, Inc. (HEB) - Still waiting for any sign of an FDA decision date out of the company on their CFS drug Ampligen. The company remains silent as the stock drips lower. |
|
|
|
|
|
|
|
|
|
|
|
 发表于 11-11-2009 05:02 PM
|
显示全部楼层
发表于 11-11-2009 05:02 PM
|
显示全部楼层
 
http://www.reuters.com/finance/s ... tamp=20091110225500
Dyer & Berens LLP Files Class Action Lawsuit Against Hemispherx Biopharma, Inc.
Tuesday, 10 Nov 2009 05:55pm EST
Dyer & Berens LLP announced that it has filed a class action lawsuit in the United States District Court for the Eastern District of Pennsylvania, Civil Action No. 09-cv-5262, on behalf of all purchasers of the securities of Hemispherx Biopharma, Inc. between February 18, 2009 and October 30, 2009, for violations of the federal Securities Exchange Act of 1934. The complaint alleges that, during the Class Period, defendants misled investors regarding the status of Hemispherx's New Drug Application (NDA) for Ampligen with the U.S. Food and Drug Administration (FDA). Specifically, defendants failed to disclose and misrepresented the fact that the FDA had requested several reports from the Company before the NDA could even be considered, thus delaying the possible approval of Ampligen by several months at a minimum. On November 2, 2009, when the Company belatedly disclosed this information, the per share price of Hemispherx's common stock dropped from $1.45 on the previous day to $1.13, a drop of more than 20%. The next day, one commenter characterized the November 2 Company update as essentially an admission that its prior public statements were false and misleading.
想不到有人代表股东们出头......
[ 本帖最后由 m.i.k.e 于 11-11-2009 05:03 PM 编辑 ] |
|
|
|
|
|
|
|
|
|
|
|

楼主 |
发表于 26-11-2009 10:51 PM
|
显示全部楼层
27/11/09
Hemispherx Biopharma, Inc. (HEB) - HEB spiked all trading day Wednesday....no news, watch it Friday |
|
|
|
|
|
|
|
|
|
|
|

楼主 |
发表于 27-11-2009 10:55 PM
|
显示全部楼层
|
|
|
|
|
|
|
|
|
|
|
 发表于 27-11-2009 11:24 PM
|
显示全部楼层
发表于 27-11-2009 11:24 PM
|
显示全部楼层
|
hebhebhebhebheb...1.4了!!!!!! |
|
|
|
|
|
|
|
|
|
|
|

楼主 |
发表于 27-11-2009 11:35 PM
|
显示全部楼层
|
|
|
|
|
|
|
|
|
|
|
 发表于 27-11-2009 11:40 PM
|
显示全部楼层
发表于 27-11-2009 11:40 PM
|
显示全部楼层
后悔没在低点进货(马后炮) |
|
|
|
|
|
|
|
|
|
|
|
 发表于 27-11-2009 11:51 PM
|
显示全部楼层
发表于 27-11-2009 11:51 PM
|
显示全部楼层
|
我本来也是要average down , 等0.5哈哈。。 |
|
|
|
|
|
|
|
|
|
|
|

楼主 |
发表于 29-11-2009 09:21 PM
|
显示全部楼层
30/11/09
Hemispherx Biopharma, Inc. (HEB) - HEB was another hot stock on Friday. The runup appears to be a short squeeze as there is no news that I can find. Resistance is located at $1.50-$1.52 as well as $1.64. |
|
|
|
|
|
|
|
|
|
|
|
 发表于 2-12-2009 08:03 AM
|
显示全部楼层
发表于 2-12-2009 08:03 AM
|
显示全部楼层
|
|
|
|
|
|
|
|
|
|
|
 发表于 2-12-2009 09:01 AM
|
显示全部楼层
发表于 2-12-2009 09:01 AM
|
显示全部楼层
结束了,漫长的等待,回来的是一纸的废话,要reject还要人家等7个月。。。
前几天买入的人会哭死吧。。。 |
|
|
|
|
|
|
|
|
|
|
|

楼主 |
发表于 2-12-2009 09:42 AM
|
显示全部楼层
今晚還會有一輪補跌。  |
|
|
|
|
|
|
|
|
|
|
|

楼主 |
发表于 2-12-2009 11:03 AM
|
显示全部楼层
|
|
|
|
|
|
|
|
|
|
|
 发表于 2-12-2009 11:10 AM
|
显示全部楼层
发表于 2-12-2009 11:10 AM
|
显示全部楼层
|
|
|
|
|
|
|
|
|
|
|

楼主 |
发表于 2-12-2009 11:15 AM
|
显示全部楼层
原帖由 m.i.k.e 于 2-12-2009 11:10 AM 发表 
我在股东们要控告 heb 上法庭时忍痛卖了, 亏整$4000. 本来以为他会跌至0.7-0.8,卖了迟点再买回......那里知道他却反弹升到1.5. 当时还在想自已真是这么倒霉.
最近發現到有一現象。。不知你有注意到嗎。
就是當一公司有問題時 股價跌了陣子後,股價沒有原因的突然飛高,過了2/3天就大跌了。。
那些BK的小金融股也是有類似的現象。。。。 |
|
|
|
|
|
|
|
|
|
|
|
 发表于 2-12-2009 11:26 AM
|
显示全部楼层
发表于 2-12-2009 11:26 AM
|
显示全部楼层
原帖由 葉芬 于 2-12-2009 11:15 AM 发表 
最近發現到有一現象。。不知你有注意到嗎。
就是當一公司有問題時 股價跌了陣子後,股價沒有原因的突然飛高,過了2/3天就大跌了。。
那些BK的小金融股也是有類似的現象。。。。
CTIC 和 HEB 让我开始研究美国医药股. 这两个股都让我亏钱, 可是也由他们开始学习买医药股. 过后都有在其他的药股赚到,包括 HGSI, INO, CVM, HLCS, IMMU, ARIA....
HEB 后来无端端从1.0 起到 1.5,的确是可疑.....
你说的我很认同,所以钱亏了,但是有学到东西还好.下次要更谨慎. |
|
|
|
|
|
|
|
|
|
| |
 本周最热论坛帖子 本周最热论坛帖子
|