|
|
 发表于 30-10-2009 01:00 AM
|
显示全部楼层
发表于 30-10-2009 01:00 AM
|
显示全部楼层
|
|
|
|
|
|
|
|
|
|
|
 发表于 30-10-2009 01:38 AM
|
显示全部楼层
发表于 30-10-2009 01:38 AM
|
显示全部楼层
|
|
|
|
|
|
|
|
|
|
|
 发表于 30-10-2009 01:46 AM
|
显示全部楼层
发表于 30-10-2009 01:46 AM
|
显示全部楼层
回复 421# 雨若情 的帖子
没错,敢敢去买多点CVM吧,橱柜加沙发都买到 |
|
|
|
|
|
|
|
|
|
|
|
 发表于 30-10-2009 10:06 AM
|
显示全部楼层
发表于 30-10-2009 10:06 AM
|
显示全部楼层
|
|
|
|
|
|
|
|
|
|
|
 发表于 30-10-2009 11:34 AM
|
显示全部楼层
发表于 30-10-2009 11:34 AM
|
显示全部楼层
|
|
|
|
|
|
|
|
|
|
|
 发表于 30-10-2009 04:58 PM
|
显示全部楼层
发表于 30-10-2009 04:58 PM
|
显示全部楼层
|
YMZ09.CBT Mini Dow Jones Indus.-$5 Dec 09 9,873.00 4:47am ET Down 30.00 (0.30%) |
|
|
|
|
|
|
|
|
|
|
|
 发表于 30-10-2009 05:12 PM
|
显示全部楼层
发表于 30-10-2009 05:12 PM
|
显示全部楼层
|
|
|
|
|
|
|
|
|
|
|
 发表于 1-11-2009 09:37 PM
|
显示全部楼层
发表于 1-11-2009 09:37 PM
|
显示全部楼层
CEL-SCI Corp. (CVM) Support & Resistance Levels - 11/2/09
Resistance Levels - $1.20, $1.45, $1.60, $1.70
Support Levels - $1.00, $0.92, $0.80
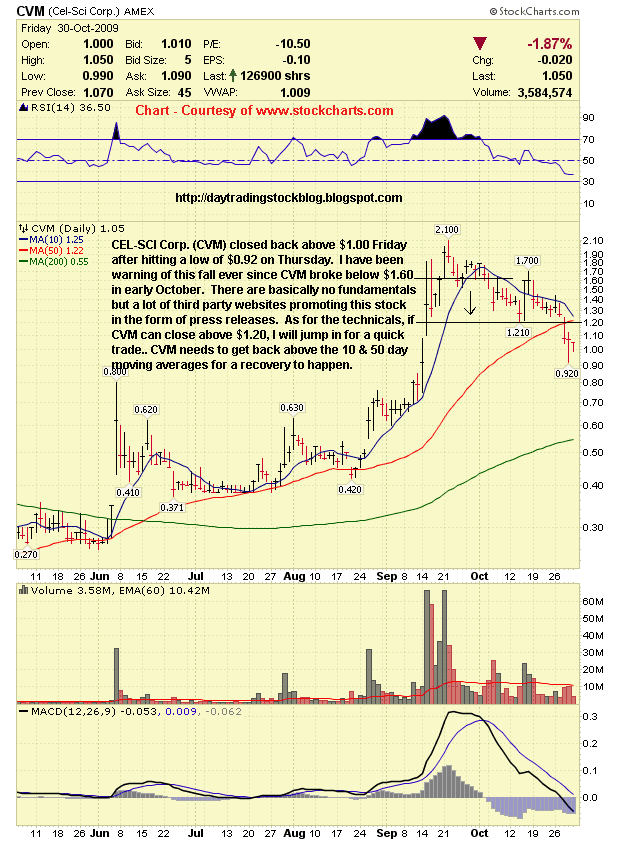
CEL-SCI Corp. (CVM) closed back above $1.00 Friday after hitting a low of $0.92 on Thursday. I have been warning of this fall ever since CVM broke below $1.60 in early October. There are basically no fundamentals but a lot of third party websites promoting this stock in the form of press releases. As for the technicals, if CVM can close above $1.20, I will jump in for a quick trade.. CVM needs to get back above the 10 & 50 day moving averages for a recovery to happen. |
|
|
|
|
|
|
|
|
|
|
|
 发表于 1-11-2009 10:34 PM
|
显示全部楼层
发表于 1-11-2009 10:34 PM
|
显示全部楼层
Discovery Laboratories Inc. (DSCO) Support & Resistance Levels - 11/2/09
Resistance Levels - $0.97, $1.00, $1.02. $1.05, $1.20
Support Levels - $0.85, $0.78, $0.65
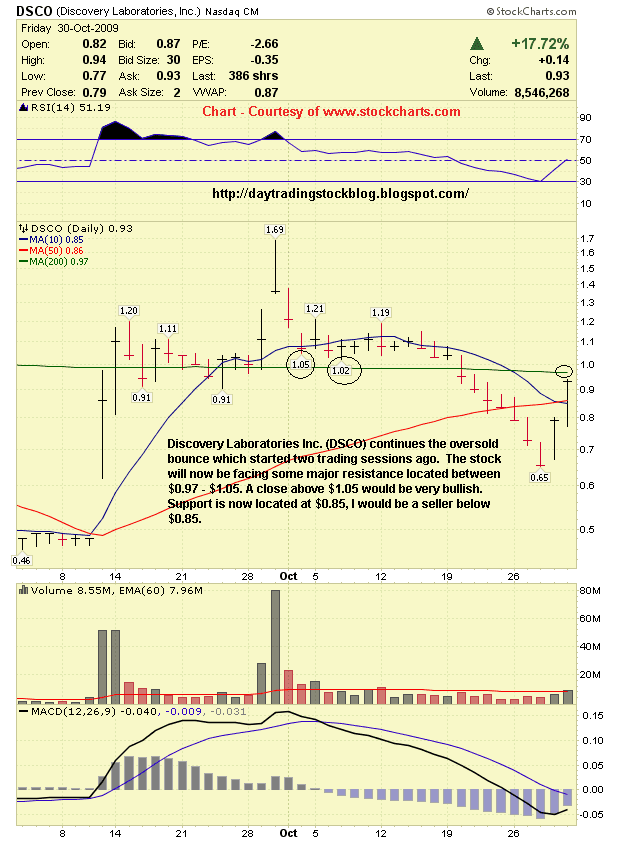
Discovery Laboratories Inc. (DSCO) continues the oversold bounce which started two trading sessions ago. The stock will now be facing some major resistance located between $0.97 - $1.05. A close above $1.05 would be very bullish. Support is now located at $0.85, I would be a seller below $0.85. |
|
|
|
|
|
|
|
|
|
|
|
 发表于 1-11-2009 11:10 PM
|
显示全部楼层
发表于 1-11-2009 11:10 PM
|
显示全部楼层
November 2nd CEOcast Weekly Newsletter
November 1, 2009 · Filed Under Stock Newsletters
CEL-SCI Corporation (AMEX: CVM), a developer of vaccines for the prevention and treatment of infectious diseases and a late-stage oncology company, announced last week that it has received more than $10 million over the past 60 days from the exercise of warrants by investors to purchase the company’s common stock. These additional funds increased the gross proceeds raised in the last 60 days to approximately $30 million, which the company has indicated that it expects to use towards its pivotal Phase III clinical trial with its cancer drug Multikine and to accelerate the development of its LEAPS compound for the treatment of H1N1 hospitalized patients. Since the beginning of the year, the company has been able to strengthen its balance sheet significantly, raising gross proceeds in excess of $43 million and eliminating its Series K convertible notes which had an initial principal value of $8.3 million. Shares lost 26 cents on the week to close at $1.05. |
|
|
|
|
|
|
|
|
|
|
|
 发表于 2-11-2009 03:13 PM
|
显示全部楼层
发表于 2-11-2009 03:13 PM
|
显示全部楼层
lupus 患者的喜讯......
Human Genome Sciences and GlaxoSmithKline Announce Positive Results in Second of Two Phase 3 Trials of BENLYSTA™ in Systemic Lupus Erythematosus
http://www.businesswire.com/port ... 1005048&newsLang=en
http://www.nytimes.com/2009/11/03/business/03lupus.html?partner=yahoofinance
Human Genome Sciences said early Monday that its experimental drug to treat lupus was effective in its second big clinical trial, raising the chances that the first new treatment for the disease in more than 40 years will make it to market.
Doctors, lupus patients and Wall Street investors have had high hopes for the drug, called Benlysta, since it succeeded in its first clinical trial in July, to almost everyone’s surprise. Before that, several other drug candidates from numerous companies had failed in treating lupus.
But to win approval to market the drug from the Food and Drug Administration, Human Genome Sciences and its partner, GlaxoSmithKline, needed a second successful trial. Having achieved that with the results announced Monday, the companies said they would file for approval of the drug in both the United States and Europe in the first half of 2010.
“The lupus community has waited for decades for one positive phase three trial of an investigative drug developed for lupus,” Dr. Joan T. Merrill, a lupus expert at the Oklahoma Medical Research Foundation and an investigator in the trial, said in a statement issued by Human Genome Sciences and GlaxoSmithKline. “Now we have two.”
Still, the drug did not perform as well in the second trial as in the first. That might temper what was expected to be a big rise in Human Genome’s stock on Monday. The biotechnology company, based in Rockville, Md., does not yet have any drugs on the market.
One recent study estimated that 322,000 Americans definitely or probably have systemic lupus erythematosus, the most common form of the disease and the one against which Benlysta was tested. Many of the sufferers are women of child-bearing age.
Lupus is an autoimmune condition, in which the body’s defense system against pathogens attacks the body’s own tissue. It can cause rashes, arthritis, mouth sores, kidney damage and other problems.
But since the symptoms wax and wane and differ from one person to another, it has been difficult to demonstrate in a clinical trial that a drug is effective against it.
In the primary measure of the drug’s effectiveness, 43.2 percent of patients taking a high dose of Benlysta had a significant improvement in their symptoms after one year, as did 40.6 percent of those taking a lower dose of the drug. By contrast, only 33.8 percent of patients getting a placebo had such an improvement.
But the difference between the results for the drug and the placebo were statistically significant only for the higher dose. In the first trial, both doses provided statistically meaningful improvements.
There were some secondary measures for which the higher high dose did not differ from placebo in a statistically significant way in the second trial.
These included the assessment of the doctors as to how their patients were doing, an index measuring the patient’s quality of life, and the proportion of patients who were able to significantly reduce their use of steroids. Steroids, which suppress the immune system, are commonly used to treat lupus but they can have severe side effects.
One reason for the difference could be that the second trial, involving 819 patients, took place primarily in North America and Europe, while the first trial had taken place in Asia, South America and Eastern Europe. It is likely that the standard of medical care in the United States and Europe is higher than in those other regions, so patients getting the placebo did relatively better.
The companies said the rate of side effects were not very different between those getting Benlysta and those getting the placebo. However, three patients getting the drug died, while none getting the placebo did.
The shares of Human Genome Sciences, which traded at $3.32 the day before the positive first trial was announced in July, closed Friday at $18.69. Analysts estimate Benlysta could eventually have annual sales of a few billion dollars.
Glaxo, a large and diversified pharmaceutical company based in London, has less riding on this drug.
Benlysta, also known as belimumab, blocks a protein that stimulates B cells, which are part of the immune system. Human Genome, a pioneer in genomics, discovered the gene for that protein. That would make Benlysta, should it be approved, one of the first drugs to arise from genomics.
[ 本帖最后由 m.i.k.e 于 2-11-2009 03:16 PM 编辑 ] |
|
|
|
|
|
|
|
|
|
|
|
 发表于 2-11-2009 04:21 PM
|
显示全部楼层
发表于 2-11-2009 04:21 PM
|
显示全部楼层
|
|
|
|
|
|
|
|
|
|
|
 发表于 2-11-2009 04:29 PM
|
显示全部楼层
发表于 2-11-2009 04:29 PM
|
显示全部楼层
|
|
|
|
|
|
|
|
|
|
|
 发表于 2-11-2009 04:32 PM
|
显示全部楼层
发表于 2-11-2009 04:32 PM
|
显示全部楼层
|
|
|
|
|
|
|
|
|
|
|
 发表于 2-11-2009 04:34 PM
|
显示全部楼层
发表于 2-11-2009 04:34 PM
|
显示全部楼层
不懂 CARI 里有几个是 HGSI 的股东?
你有再买回吗? |
|
|
|
|
|
|
|
|
|
|
|
 发表于 2-11-2009 04:35 PM
|
显示全部楼层
发表于 2-11-2009 04:35 PM
|
显示全部楼层
原帖由 m.i.k.e 于 2-11-2009 04:34 PM 发表 
不懂 CARI 里有几个是 HGSI 的股东?
你有再买回吗?
沒有。 |
|
|
|
|
|
|
|
|
|
|
|
 发表于 2-11-2009 04:55 PM
|
显示全部楼层
发表于 2-11-2009 04:55 PM
|
显示全部楼层
原帖由 葉芬 于 2-11-2009 04:35 PM 发表 
沒有。  |
|
|
|
|
|
|
|
|
|
|
|
 发表于 2-11-2009 05:03 PM
|
显示全部楼层
发表于 2-11-2009 05:03 PM
|
显示全部楼层
|
|
|
|
|
|
|
|
|
|
|
 发表于 2-11-2009 05:38 PM
|
显示全部楼层
发表于 2-11-2009 05:38 PM
|
显示全部楼层
来了! 来了 !

Human Genome Sciences, Inc. (HGSI) Pre-Market Trading
Human Genome Sciences, Inc.
Pre-Market
Last: | $ 23 | Pre-Market
High: | $ 23 | Pre-Market
Volume: | 6,100 | Pre-Market
Low: | $ 21.88 |
Pre-Market
Time (ET) | Pre-Market
Price | Pre-Market
Share Volume | | 04:35 | $ 23 | 400 | | 04:34 | $ 23 | 200 | | 04:34 | $ 23 | 400 | | 04:19 | $ 21.88 | 200 | | 04:18 | $ 21.88 | 1,000 | | 04:17 | $ 21.88 | 500 | | 04:15 | $ 21.88 | 400 | | 04:15 | $ 21.88 | 3,000 |
|
|
|
|
|
|
|
|
|
|
|
|
 发表于 2-11-2009 09:38 PM
|
显示全部楼层
发表于 2-11-2009 09:38 PM
|
显示全部楼层
还要等一个小时.....煎熬啊.....
要办个可以 trade pre-market 的平台才行.
讲了整半年,都还没有去办 etrade 或 ameritrade. |
|
|
|
|
|
|
|
|
|
| |
 本周最热论坛帖子 本周最热论坛帖子
|