|
|
 发表于 5-1-2011 09:51 PM
|
显示全部楼层
发表于 5-1-2011 09:51 PM
|
显示全部楼层
暫時Pre-Market最強是
1) OPXA
2) XING
3) PEIX |
|
|
|
|
|
|
|
|
|
|
|
 发表于 5-1-2011 09:54 PM
|
显示全部楼层
发表于 5-1-2011 09:54 PM
|
显示全部楼层
回复 葉芬
ANX和SSN还没卖不敢买进...但会继续观察..价钱已很吸引了...
SUNNY仔 发表于 5-1-2011 05:02 PM 
SSN 漲勢還很強。
要是能再加上好消息,股價2元沒問題。 |
|
|
|
|
|
|
|
|
|
|
|
 发表于 5-1-2011 10:48 PM
|
显示全部楼层
发表于 5-1-2011 10:48 PM
|
显示全部楼层
本帖最后由 migo 于 5-1-2011 10:53 PM 编辑
回复 2322# 葉芬
注意ARNA 来势凶凶
CHGS迟来的鸟没虫吃 |
|
|
|
|
|
|
|
|
|
|
|
 发表于 6-1-2011 12:35 AM
|
显示全部楼层
发表于 6-1-2011 12:35 AM
|
显示全部楼层
本帖最后由 葉芬 于 6-1-2011 12:49 AM 编辑
PZG 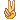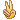
SSN 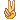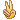
PZG 只是調整2天而已。 |
|
|
|
|
|
|
|
|
|
|
|
 发表于 6-1-2011 08:11 AM
|
显示全部楼层
发表于 6-1-2011 08:11 AM
|
显示全部楼层
回复 2323# migo
买来玩玩的420UNIT ARNA出货了..赚了五十多蚊美元买汉宝包吃... |
|
|
|
|
|
|
|
|
|
|
|
 发表于 6-1-2011 08:16 AM
|
显示全部楼层
发表于 6-1-2011 08:16 AM
|
显示全部楼层
回复 2324# 葉芬
开市时金价掉,还以为PZG会继续探低的...结果却是反弹... |
|
|
|
|
|
|
|
|
|
|
|

楼主 |
发表于 6-1-2011 08:21 AM
|
显示全部楼层
回复 2325# SUNNY仔
你的汉堡相当贵一下.... |
|
|
|
|
|
|
|
|
|
|
|

楼主 |
发表于 6-1-2011 08:24 AM
|
显示全部楼层
本帖最后由 roberto 于 6-1-2011 08:38 AM 编辑
press release
Jan. 5, 2011, 7:00 p.m. EST
Samson Oil & Gas Updates Operations & Comments on Market Activity
DENVER, Jan05, 2011 (BUSINESS WIRE) -- Samson Oil & Gas Limited SSN 1.56, -0.02, -1.27%) advises on recent operational activity:
NORTH PLATTE3-D SEISMIC SURVEY
Acquisition operations have been completed in the North Platte 3-D seismic project. This acquisition encompassed 60.4 square miles and will assist the appraisal of the area by the identification of naturally occurring fractures in the Cretaceous Niobrara Formation and conventional structural/stratigraphic targets that are productive in the area.
The acquisition operations went smoothly with some minor downtime experienced because of high velocity winds, which would have caused ambient noise levels to be exceeded during recording.
The data has been progressively loaded into a Denver-based seismic processing house and the first products from this processing are expected to be available to Samson by mid January.
NORTH STOCKYARD OILFIELD
The fracture stimulation operations for the Earl and Rodney wells that were expected to be performed in December are now expected to be concluded during the first quarter of 2011. This delay is unfortunate and has been caused by the heightened level of activity in the Williston Basin.
Samson is planning to enter into a long-term contract for the provision of both drilling and fracture stimulation services so that these delays are not replicated in its Goshen County project.
RECENT MARKET ACTIVITY
Samson also provides advice concerning the recent increase in trading volumes and prices of Samson's ordinary shares, which are traded on the Australian Stock Exchange, and its American Depositary Shares, representing twenty ordinary shares, which trade in the U.S. on NYSE Amex.
"Samson is not aware of any recent corporate developments to account or this activity," said Samson's Managing Director Terry Barr, "We are aware, however, that an oil and gas consulting company engaged by Samson to provide investor relations and other services, did publish a report on Samson dated December 29, 2010, which was an update of its prior report on the company.Though Samson cannot comment on the accuracy of that estimate or any of the other information in the report, we are also aware that the new report estimated Samson's net asset value per share to be significantly greater than current market prices for its shares,"
The report also discusses the considerable activity being undertaken by Samson's competitors in the Denver-Julesburg Basin with respect to the development of the Niobrara Formation, including credible initial production rates, and comments on the prospects for this play becoming an important oil producing unit.
While Samson did not participate in the preparation or publication of the report and is not responsible for the accuracy or quality of any the statements, information or conclusions contained therein, a copy of the report has been posted on Samson's website, www.samsonoilandgas.com ,for the convenience of Samson's shareholders and other interested parties. |
|
|
|
|
|
|
|
|
|
|
|
 发表于 6-1-2011 09:13 AM
|
显示全部楼层
发表于 6-1-2011 09:13 AM
|
显示全部楼层
回复 葉芬
开市时金价掉,还以为PZG会继续探低的...结果却是反弹...
SUNNY仔 发表于 6-1-2011 08:16 AM 
所以說股市是不能預測的,特別是美股市。  |
|
|
|
|
|
|
|
|
|
|
|
 发表于 6-1-2011 09:15 AM
|
显示全部楼层
发表于 6-1-2011 09:15 AM
|
显示全部楼层
回复 SUNNY仔
你的汉堡相当贵一下....
roberto 发表于 6-1-2011 08:21 AM 
美金的漢堡包當然是貴些的。
但 美味可口。 |
|
|
|
|
|
|
|
|
|
|
|
 发表于 6-1-2011 09:30 AM
|
显示全部楼层
发表于 6-1-2011 09:30 AM
|
显示全部楼层
本帖最后由 葉芬 于 6-1-2011 10:16 AM 编辑
press release
Jan. 5, 2011, 7:00 p.m. EST
Samson Oil & Gas Updates Operations & Comments on Market ...
roberto 发表于 6-1-2011 08:24 AM 
AUS-SSN 看了這新聞,股價跌了0.003 (-4.05%)
After Hours SSN-US 跌了8%,不過只有5000Shares 交易。還好。還好。
又給你說中,SSN不出新聞還好過出新聞。 |
|
|
|
|
|
|
|
|
|
|
|
 发表于 6-1-2011 10:16 AM
|
显示全部楼层
发表于 6-1-2011 10:16 AM
|
显示全部楼层
回复 2325# SUNNY仔
恭喜呀!什么汗宝这么贵!我吗最近很忙.没时间玩投机!今天买了HDY7500 UNIT.
看看能赚点小钱!看来ARNA还是有希望的. |
|
|
|
|
|
|
|
|
|
|
|
 发表于 6-1-2011 10:18 AM
|
显示全部楼层
发表于 6-1-2011 10:18 AM
|
显示全部楼层
回复 SUNNY仔
恭喜呀!什么汗宝这么贵!我吗最近很忙.没时间玩投机!今天买了HDY7500 UNIT.
看 ...
migo 发表于 6-1-2011 10:16 AM 
前天我有貼上來說 HDY又要來了。 |
|
|
|
|
|
|
|
|
|
|
|
 发表于 6-1-2011 02:37 PM
|
显示全部楼层
发表于 6-1-2011 02:37 PM
|
显示全部楼层
AUS - SSN
0.074 0.000 (0.00%)
Jan 6 - Close
ASX data delayed by 20 mins
澳洲的SSN平盤。
就看今晚美國的SSN表演了。 |
|
|
|
|
|
|
|
|
|
|
|
 发表于 6-1-2011 10:19 PM
|
显示全部楼层
发表于 6-1-2011 10:19 PM
|
显示全部楼层
|
sprint nextel 的vol也算高吗??有潜能吗? |
|
|
|
|
|
|
|
|
|
|
|
 发表于 6-1-2011 10:20 PM
|
显示全部楼层
发表于 6-1-2011 10:20 PM
|
显示全部楼层
回复 2327# roberto
昨晚去探望以为前辈,她刚从英国回来,说英国的MC一套要价27块英镑 ,至于美国的,加水钱精神精力的,差不多啦.. ,至于美国的,加水钱精神精力的,差不多啦.. |
|
|
|
|
|
|
|
|
|
|
|
 发表于 6-1-2011 10:22 PM
|
显示全部楼层
发表于 6-1-2011 10:22 PM
|
显示全部楼层
|
sprint nextel 的vol也算高吗??有潜能吗? |
|
|
|
|
|
|
|
|
|
|
|
 发表于 6-1-2011 10:23 PM
|
显示全部楼层
发表于 6-1-2011 10:23 PM
|
显示全部楼层
回复 2334# 葉芬
SSN-US PRE-MART掉了...起码调整5%... |
|
|
|
|
|
|
|
|
|
|
|
 发表于 6-1-2011 10:25 PM
|
显示全部楼层
发表于 6-1-2011 10:25 PM
|
显示全部楼层
本帖最后由 SUNNY仔 于 6-1-2011 10:26 PM 编辑
ANX PRE-MART 狂起接近30%...
芬姐米哥,有什么意见?卖还是等$4... |
|
|
|
|
|
|
|
|
|
|
|
 发表于 6-1-2011 10:30 PM
|
显示全部楼层
发表于 6-1-2011 10:30 PM
|
显示全部楼层
|
结果在排着$3.55...因为记起芬姐的签名...看可否套利先...哈哈 |
|
|
|
|
|
|
|
|
|
| |
 本周最热论坛帖子 本周最热论坛帖子
|